Clinic visit notes
I went to the ALS clinic on Monday, May 2, and these are my notes.
Forced vital capacity, the test sine qua non, was 96 percent (92, 93, 96). On 1/28/2004 when they confirmed my diagnosis, it was 94 percent. So, holding steady.
My inhalation force was over 120. The dial only goes up to 120. In April of 2004 it was 115.
My peak cough flow rate was 600, which is down from the 700 of April 2004 ... but I woke up yesterday morning with a sore throat which may be due to a cold but which is probably allergies -- and so, I didn't want to cough very hard. My body nixed the idea.
My weight was 138 pounds. Blood pressure 149/85, but I was stressing that I might have offended the technical guy, so that probably explains that.
They also have this test of saying animal names (any that you can think of). I said 26, which they said was fine. In April 2004 I said 25. But you know, this time I got about 75 percent to the time limit and then just stopped, because it seemed so silly to be standing there saying "Uh, kuala, uh, piraña, sloth..." I didn't even go into reptiles or sea life.
My blood oxygen level was 98 percent, which they found by using that cool laser-based scanner. That's good, I'm told, but blood oxygen level is one of the last things interfered with by ALS, so don't obsess on it.
They seemed to find the creatine before bed idea novel and worthy. I hope it helps someone else. They also suggested drinking tonic water before bed, because the quinine helps reduce cramping.
They also suggested Robitussin DM to reduce cramping, but I ain't going there.
They want to get me to try Namenda again. They said it would help reduce cramping, and be a good stand-in instead of riluzole. But it may make me a bit more loose and stumbly, so I need to be extra careful. They hadn't known I'd already tried Namenda. I told them about the forgetfulness I had experienced, and they said that didn't sound related. The clinic doctor wants me to take the entire daily dose at night, instead of splitting it between morning and night. So I started that last night. They even gave me a brand-new starter kit. I'd gotten halfway through the other one before bailing out. The doctor said the risk with Namenda is constipation. She said it may make me more wobbly for a while, but that it might even help control the "lability" (inappropriate laughing).
The people from ALSA and MDA are wonderful, as usual.
They gave us a number of small aids to help with gripping things, plus a number of pamphlets.
There really ought to be a way to make a .WAV file on a Macintosh, I just don't know how. The purpose would be to feed my own voice, at some point, into a speech device.
We also talked about Medicare, Medigap (plans A through J!), and how to make that COBRA transition which I soon will, given that my employer was supposed to terminate me May 1 (due to extended long-term disability status).
They repeated again: Always elect part A and part B of Medicare, even though you do have to pay for part B.
They also urged me to go to the physical therapist and do range of motion exercises. I will.
And we talked to the clinic director, who listened to my lovely wife's brilliant and compassionate suggestion, that since we know (all too well) how untreated ALS behaves, why not use that data as the control group, and give everyone in an ALS clinical trial the drug? I added that you would get higher participation.
He said they were already pushing for that, but the FDA or NIH is currently unwilling. There is apparently some such arrangement for cancer, though.
The ceftriaxone clinical trial, we were told, has been postponed because the FDA wants more data on the effect of long-term dosing in animals. The trial is expected to start in September or October.
As a former journalist, I can tell you that I think this would make a good news story. The irony is vast. Maybe some of you have suggestions on how to use this delay as part of an awareness campaign to get the FDA to create a new, alternate, procedure for ALS clinical trials? I mean, we don't have a voice -- because there are so few of us! Because most of us die so fast. The FDA should ask the people who are in their graves now whether those people would be willing to risk taking this FDA-approved drug without more months of animal studies. The FDA seems to be most interested in making sure they have no blood on their hands. But in so doing, they get corpses.
I once again begged for a database to run queries against, and was told that NIH has one called NINDS (NIHNDS?) which is available to the public, though I may have to request access.
We talked about ALS pathways. Some of these are believed to be:
- glutamate toxicity
- calcium toxicity thought to occur when excess glutamate brings calcium into the neuron
- neuro-inflamation (Not gross. It won't show on an MRI)
- apoptosis
- SOD1 mutation (familial)
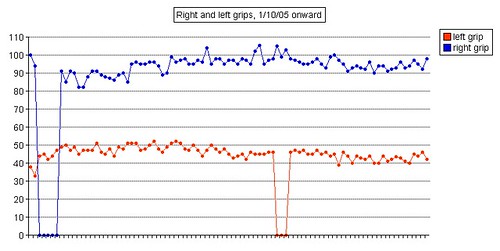
Left grip is 42 pounds (38, 42, 40), right grip is 98 pounds (93, 98, 92), left leg balance is 7.43 seconds, and inhale volume is 4750 mL.

<< Home